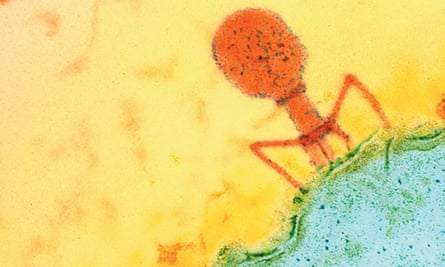
It is, say enthusiasts, the cure that the world forgot. An old therapy that could take on the new superbugs.
Discovered in 1917 by French Canadian biologist Félix d’Hérelle, phages – or bacteriophages – are tiny viruses that are natural predators of bacteria. In many countries they were supplanted during the second world war by antibiotics but continued to be used for decades in eastern Europe.
They are now being seen by some scientists as a complement – and perhaps an alternative –to antibiotics, the overuse of which has led to increasing bacterial resistance and the advent of the superbug.
Tobi Nagel, a California-based biomedical engineer, launched Phages for Global Health (PGH) in 2014 to help developing countries fight antimicrobial resistance (AMR). She had become disillusioned with the inequality in the pharmaceutical industry, in which she worked for 15 years, and says: “We have 30 years until the worst of this crisis. Phages could be made into drugs in less than 10 years.
“I was becoming increasingly frustrated that the drugs I was working on in the US, which typically cost $1bn to develop, were not accessible to most people living in developing countries.”
Unlike antibiotics, phages must be used in a highly targeted way, because each phage is effective against only a limited number of bacteria. They have, says Nagel, undergone important therapeutic and commercial development.
“In the near future, phages will be secondary to antibiotics as they can still work against most pathogens. Phages will be the last option when you have no choice,” says Sivachandran Parimannan, a researcher at the Centre of Excellenceat AIMST University in Kedah, Malaysia.
According to the World Health Organization (WHO), antimicrobial resistance is a rising threat to global health, jeopardising decades of medical progress and transforming common infections into deadly ones. A UN report published last year suggested yearly deaths from drug-resistant diseases could rise from the current 700,000 to 10 million in 30 years if no action is taken.
“In principle, phages are cheaper and quicker to develop than conventional drugs, can be designed to minimise future bacterial resistance and have no reported side-effects,” says Nagel. “They can be produced with relatively simple equipment that is readily available to scientists in developing countries, which are the most endangered by the rise of AMR.”
According to a 2014 study commissioned by British authorities, by 2050 approximately 90% of deaths attributable to AMR are expected to occur in Africa and Asia.
Research in phage therapy, relaunched over the past 10 years in Europe and the US, is still in its infancy in developing countries. Phage for Global Health hopes to promote a transfer of skills and knowledge.
There are downsides – phages are slower than antibiotics. Not readily available, they cannot be used in an emergency setting and time is usually needed to find the right phage to target the relevant bacteria. They have a narrow spectrum and are less stable than chemical drugs.
Phage therapy tends to be used in a personalised way which makes comparisons difficult and it is likely they are more efficient against certain bacteria, while antibiotics are more efficient against others, so new studies suggest it is better to combine both. Phage therapy centres such as the ones that exist in Poland and Georgia claim to have a success rate of 75-85%.
More research is needed to know if phage use has any negative effect on the human body, but so far few side-effects have been reported.
“There are specific needs in developing nations. Even common bacterial infections will have their own strains associated with specific countries,” says Martha Clokie, professor of microbiology at the University of Leicester and a trainer with PGH. “For example Salmonella food poisoning is a problem worldwide, but each African country will likely have different strains of the bacteria and therefore need specific phages.”
Since 2017, four two-week workshops have been organised in Africa, training about 100 scientists who have passed on what they have learned to more than 1,000 students.
A fifth workshop planned for Malaysia has been postponed to next year due to the pandemic. The global disruption has led Nagel to shift some activities online. Part of the learning material will be soon available on the website phage.directory thanks to a grant from the Mozilla Foundation.
“The training in Malaysia will sensitise more researchers from south-east Asian countries to the use of phages, which is not limited to human health but has applications for agriculture, livestock and food,” says Heraa Rajandas, a lecturer at AIMST, which will host the event.
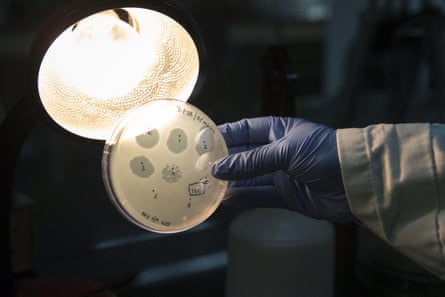
At the end of the workshops, trainees know how to take phages from nature, isolate those corresponding to the target bacteria and characterise them, making use of DNA sequencing, to ensure that the phage does not have undesirable genetic properties.
Due in part to a lack of technological, financial and human resources, scientific teams working in the global south often focus more on animal and plant health, as well as food decontamination.
“The use of antibiotics by Ugandan farmers is massive, and this is a selection pressure for resistant organisms which can get to the human beings,” says Jesca Nakavuma, a microbiologist at Makerere University in Kampala who hosted the first PGH training in 2017. With funding from the African Union, she is working on phage cocktails that act against fish pathogens for aquaculture and now hopes to market them.
“There are also other ongoing projects from former participants on bovine mastitis, crop pathogens and on bacteria which are multidrug resistant such as E. coli, Klebsiella and Pseudomonas,” says Clokie.
PGH is also coordinating two transnational research programmes involving scientific institutions from Europe, North America and Africa. “In Kenya, two teams I collaborate with are working on phage cocktails against Campylobacter and Salmonella to decontaminate poultry meat, which is the cause of many food-borne infections,” says Nagel. The second project aims to test cholera phages in Bangladesh and then apply this treatment in the Democratic Republic of the Congo.
While producing phage-based drugs for food decontamination is already possible in some countries, the development of phage therapy for human health faces bigger hurdles. The lack of clinical trials meeting international standards means that – outside the former Soviet bloc – access to phages is either nonexistent or restricted to compassionate use. Many countries lack an appropriate regulatory framework.
In western countries, the manufacture of medicines must comply with strict criteria before they reach the market. “GMP standards can increase the production cost of a drug by 10 times; this is a major obstacle for all players. There are other forms of controlled production which might enable developing countries to access drugs they desperately need at prices they can actually afford,” says Nagel, who, with several colleagues, has outlined the role the WHO could potentially play in overseeing the use of phage-based products in developing nations.
The WHO has not officially included phage therapy in its action plan against antibiotic resistance, but Nagel hopes this could change if new clinical trials prove positive.


No comments:
Post a Comment